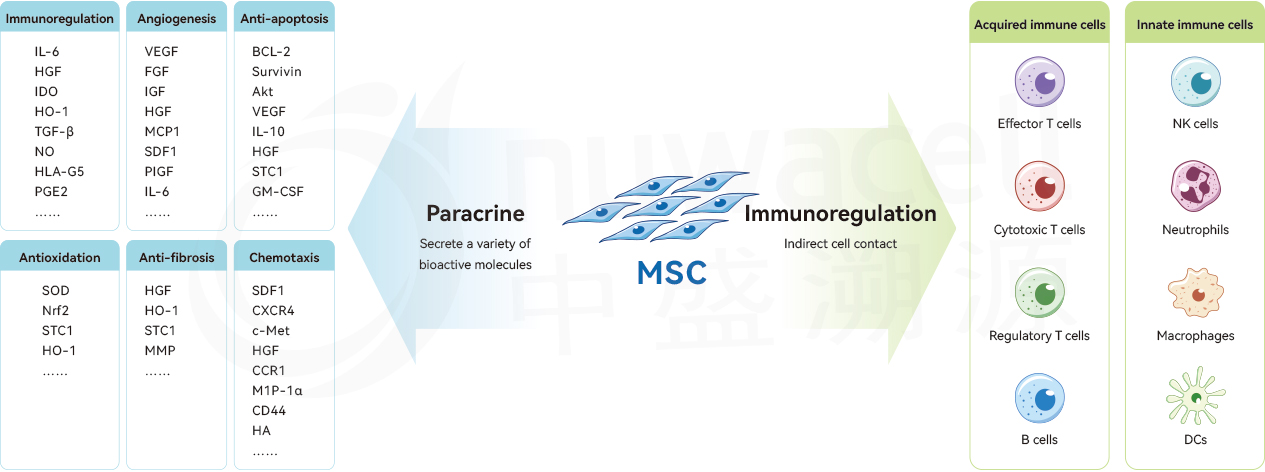
The Mechanism of Action of MSC
Moreover, MSCs do not express major histocompatibility complex (MHC) class II molecules, and they have low immunogenicity. Allogeneic transplantation of MSCs does not trigger immune rejection reactions.
Current clinical research indicates that MSCs have demonstrated remarkable potential in the treatment of a variety of diseases, particularly in the fields of autoimmune diseases and tissue-injury diseases. With the continuous deepening of research, MSCs are expected to play a crucial role in the treatment of more diseases.
Induced pluripotent stem cells (iPSCs) possess the characteristics of unlimited expansion and easy genetic engineering modification. The generated induced mesenchymal stem cells (iMSCs) are all derived from the same seed iPSC cells, featuring large-batch production and more stable and uniform quality of the obtained iMSCs. Meanwhile, by carrying out genetic modification at the iPSC stage, iMSCs with specific functions can be obtained. More importantly, iMSCs induced and differentiated from iPSCs are more similar to MSCs in the fetal stage and are the "youngest" MSCs with the strongest proliferation ability and complete differentiation potential.
Therefore, it can be said that the iPSC technology is an important innovative direction for the development of "off-the-shelf" MSC therapies.
"NCR100" for the treatment of knee osteoarthritis (the first iPSC-derived MSCs approved for clinical trials in China), Phase II clinical trial.
"NCR102" for steroid-refractory acute graft-versus-host disease, Phase II clinical trial.
"NCR101" for the treatment of interstitial lung disease (the first globally approved iPSC-derived genetically modified MSCs for clinical trials), Phase I clinical trial.


 Overview
Overview Anti-inflammatory and Tissue Repair
Anti-inflammatory and Tissue Repair Regenerative Medicine
Regenerative Medicine Immunotherapy
Immunotherapy