


Malignant tumors have emerged as one of the leading causes of death in China, with the number of cases rising steadily each year. Currently, effective treatment options remain limited. Recent advancements in tumor immunotherapy have shown significant progress. By enhancing the patient's immune system to combat tumors, this approach offers new therapeutic options and robust adjuvant treatments for patients who have not responded well to traditional anti-tumor therapies.
NK (Natural Killer) cells are innate immune cells capable of nonspecific killing tumor cells without prior sensitization. They recognize tumor cells through a series of activating and inhibitory receptors on their surface. Upon recognition, NK cells form immune synapses with tumor cells, releasing cytotoxic molecules such as perforin and granzyme to induce target cell death. Additionally, NK cells secrete various cytokines, chemokines, and growth factors, facilitating the coordinated attack on tumor cells by other immune cells.
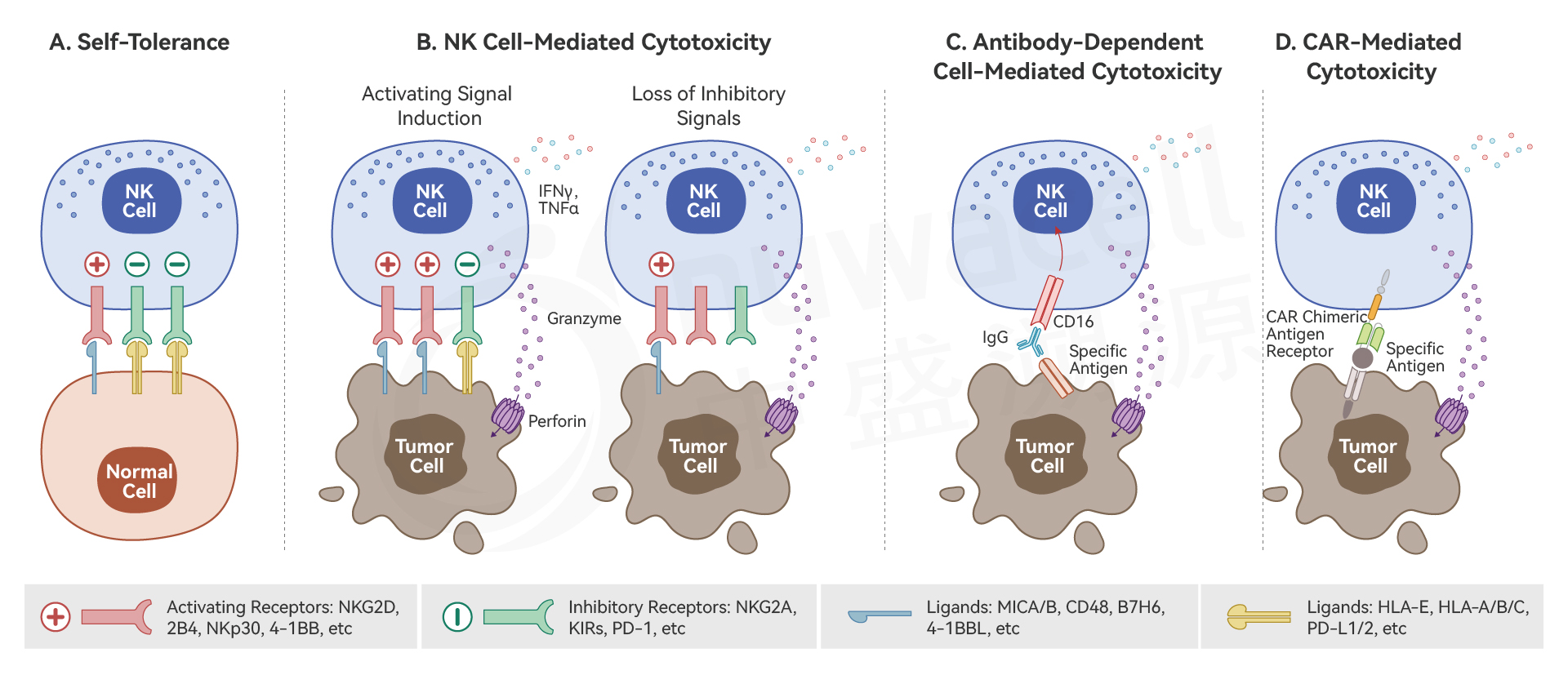
The Mechanism of NK Cells in Killing Tumor Cells
Compared to CAR-T (Chimeric Antigen Receptor T-cell) therapy, NK cells exhibit a significantly lower risk of graft-versus-host disease (GvHD) and cytokine release syndrome (CRS), offering a safer profile. NK cells can be administered in single or multiple doses, making them a promising off-the-shelf immune cell therapy with high accessibility and broad applicability across various malignant tumors.
Induced pluripotent stem cells (iPSCs) possess the potential for unlimited expansion, differentiation into all three germ layers, and ease of genetic modification. Unlike NK cells derived from peripheral blood or umbilical cord blood, iPSC-derived NK cells offer advantages such as large-scale production, consistent quality, superior tumor-killing efficacy, and resilience to freeze-thaw cycles. These cells also allow for complex genetic engineering. Utilizing iPSCs as a stable cell source enables the production of highly homogeneous, cost-effective, and convenient NK cell therapies tailored to diverse tumor treatment needs.
“NCR300” for the Treatment of Myelodysplastic Syndrome (the First iPSC-derived NK Cell Product Approved for Clinical Trials in China) ——Phase I Clinical Trial
“NCR300” for the Prevention of AML Relapse after allo-HSCT——Phase I Clinical Trial